30 Jul 2025
Silver Recovery

Silver and Original Photo Paper
Silver is an essential component for the quality of prints on Original Photo Paper. Silver is part of the photographic layer, embedded in gelatine. When the Silver (Silver Halide Crystals) in the Photographic paper is exposed, a latent image is created by means of an RGB exposure system. The photographic paper is then put through an RA4 development process using a developer and a bleach-fix to create the image on the paper. During this process, cyan, magenta, and yellow dyes are formed in three different layers.
How do we recover that silver out of the paper?
During the RA4 development process, approximately 99% of the silver present in the photo is removed. But where does it all go?
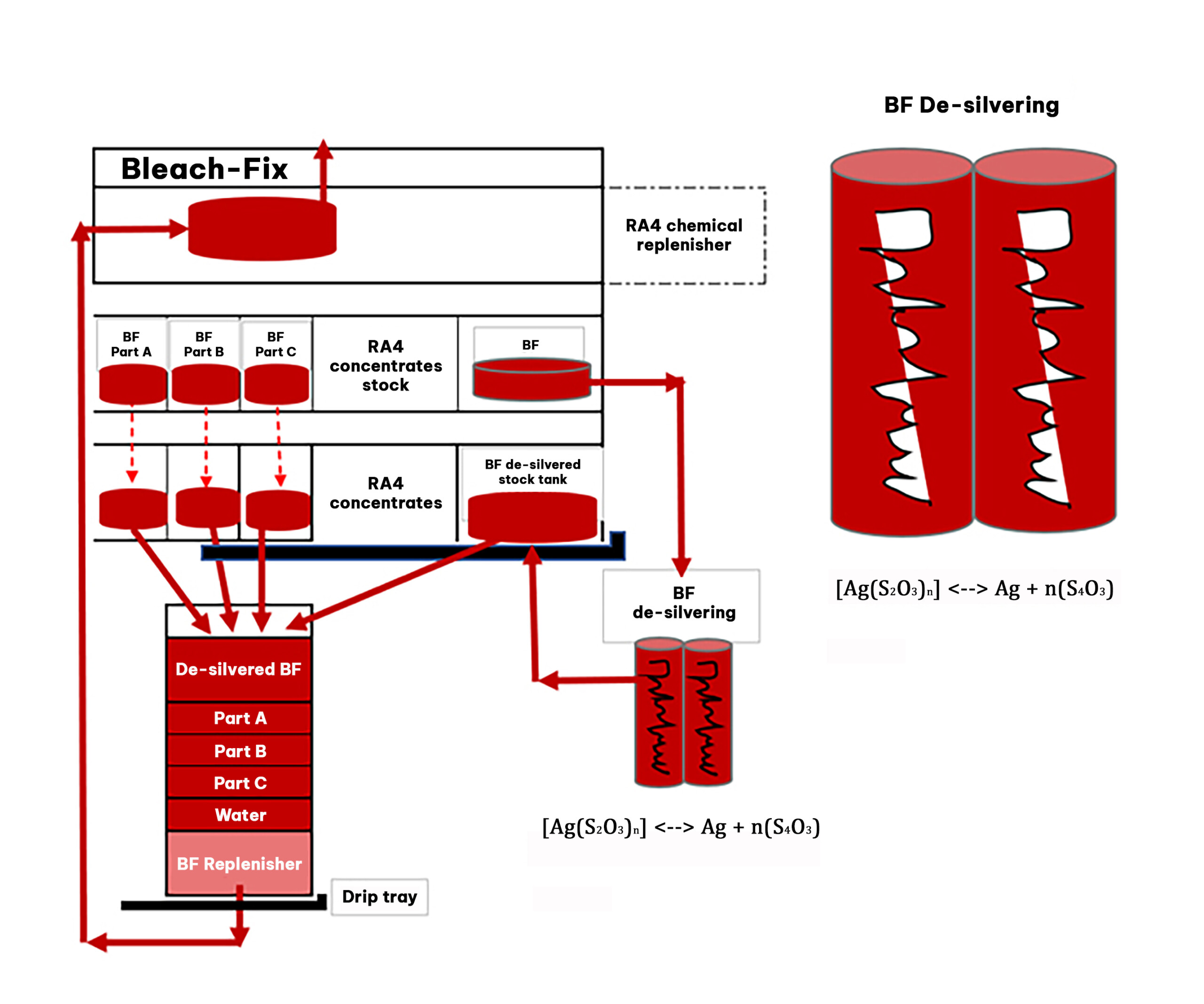
Most of this silver, around 96%, ends up in the bleach-fix solution, where it combines with thiosulphate ions to form complexes. This silver can be recovered in-house by most large photo labs using electrolytic de-silvering. Some photo labs may choose to collect the chemical waste to be handled by a waste management company.
The remaining 3% of the silver from the RA4 development process ends up in the wash water. To prevent this silver from being lost down the drain some photo labs employ an ion-exchange de-silvering system. This system utilizes ion-exchange resins that selectively capture silver ions from the wash water, allowing for their recovery and re-use. This in-house de-silvering process ensures that the silver is properly managed instead of being handled by a regional water treatment plant.
So far, the easiest part of silver recovery. Now let’s dive into the technical part:
Electrolytic silver recovery from a thiosulfate-based bleach-fix
Silver is present in the bleach-fix solution in the form of complexes with thiosulfate ions, which can dissociate in equilibrium to release silver ions and thiosulfate ions.
At the cathode, which is a negatively charged electrode, the silver ions are reduced by gaining electrons to form solid silver. The reaction can be represented as: Ag + + e –> Ag.
At the anode, which is a positively charged electrode, several reactions can occur.
Important reactions at the anode are the oxidation of thiosulphate and sulphite. Additionally at the cathode the reduction of iron takes place.
These reactions collectively contribute to the reduction in the concentration of thiosulphate, sulphite, and pH in the bleach-fix solution. The effectiveness of the bleach-fix solution can be restored by aeration, which converts inactive Fe2+ ions into active Fe3+ ions.
To ensure effective de-silvering, sufficient agitation is required to prevent the formation of silver sulphide on the cathode.
Gelatin is only desirable up to about 2% of the silver weight as excess can also lead to sulphiding.
It is important to note that de-silvering in bleach-fix solutions is more challenging compared to fixer recovery, due to the involvement of iron III and iron II reactions. To counteract the reoxidation of iron, a high current system is typically employed.
A less commonly used alternative continuous de-silvering system is the PSR (Ilford) Electrogen. This system employs the controlled reduction of iron III to iron II using sodium dithionite. To ensure precise dosing, a probe and dosing unit are utilized to prevent the addition of excess reagent. Adding excessive amounts of sodium dithionite can result in precipitation of silver. Conversely, if there is a deficiency of sodium dithionite, the plating process may not occur properly or at all, especially since the plating unit is designed with a low current density.
Electrolytic Silver Recovery :
Silver recovery rates & efficiency
By applying Faraday’s law, these calculations provide a way to estimate the potential silver recovery and efficiency in electrolytic de-silvering.
Mass of substance = (Current × Time × Equivalent mass) / (Faraday’s constant)
Example calculation Potential recovery: 100 A *3600 s *107.87 / 96485 = 100 A*4,02 = 402 g Ag per Amp hour
- Potential recovery (grams of Ag per Amp hour) = current x 4,02
- Theoretical recovery %= (Potential recovery / Ag in) x 100
- Actual Recovery % = (Ag_out / Ag_in) x 100
- Efficiency % = (Actual Recovery / Theoretical Recovery) x 100